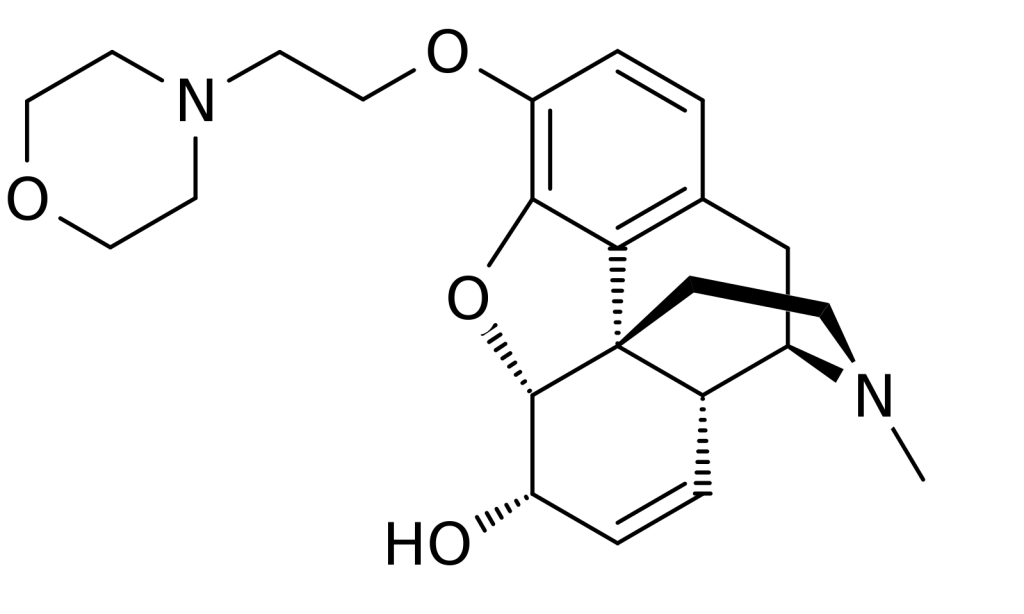
Pholcodine Monohydrate is used as the primary active ingredient in the formulation of antitussive (cough suppressant) medications. This drug is mainly prescribed for the relief of dry, irritating, non-productive coughs. It acts centrally on the brain to suppress the cough reflex.
Pholcodine is a semi-synthetic opioid alkaloid that reduces the cough reflex by acting on central cough receptors in the medulla oblongata. Compared to codeine and other opioids, it carries a lower risk of addiction and dependence and is less likely to cause respiratory depression. The onset of action is typically within 30 to 60 minutes after oral administration, with effects lasting up to 6 hours.
Montelukast, marketed under the brand name Singulair, is a widely used leukotriene receptor antagonist (LTRA) that plays a critical role in the management of respiratory and allergic conditions. Approved by the U.S. Food and Drug Administration (FDA) since 1998, montelukast is prescribed globally for the prevention and long-term treatment of asthma, relief of symptoms of seasonal allergic rhinitis, and prevention of exercise-induced bronchospasm.
Montelukast is particularly valued for its oral administration route, making it a convenient option for patients of all ages, including children. It is often used as an adjunct therapy in patients whose asthma is not adequately controlled with inhaled corticosteroids alone. Montelukast works by selectively blocking leukotriene D4 receptors in the lungs and bronchial tubes.
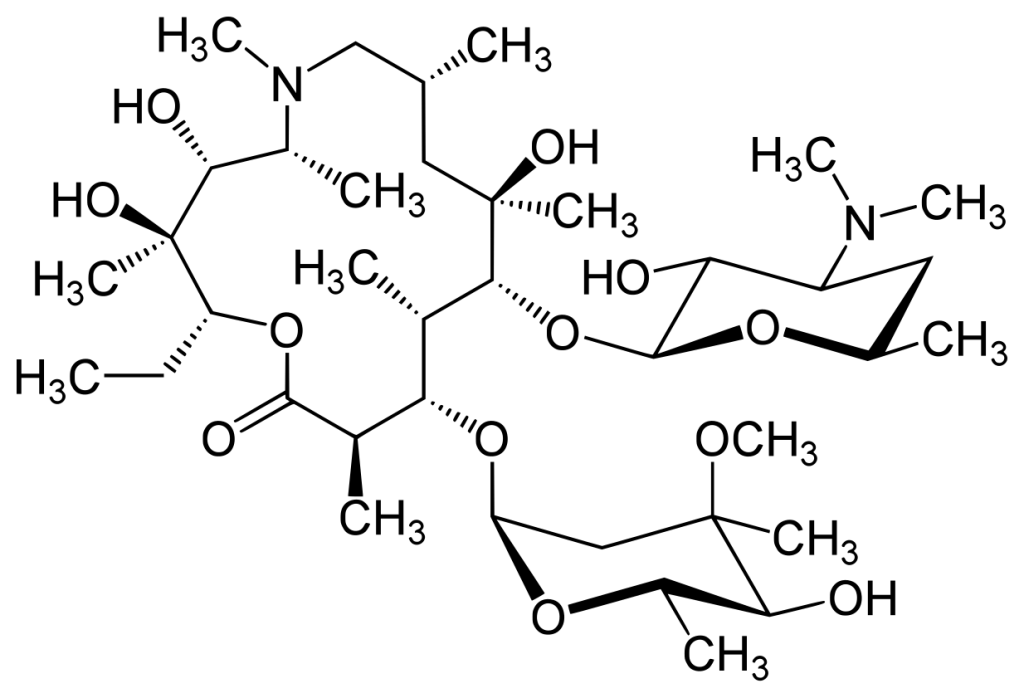
Azithromycin serves as the active pharmaceutical ingredient in both oral and injectable azithromycin medications. As a member of the macrolide class of antibiotics, it is prescribed for the treatment of a wide range of bacterial infections, including upper and lower respiratory tract infections (such as bronchitis and pneumonia), ear, nose, and throat infections, skin and soft tissue infections, and certain sexually transmitted infections.
Azithromycin works by inhibiting bacterial protein synthesis through binding to the 50S ribosomal subunit, thereby halting bacterial growth and reproduction. Its long half-life allows for once-daily dosing and a short treatment duration (typically 3 to 5 days). Compared to other macrolides, azithromycin offers better gastrointestinal tolerability, and its side effects are generally mild.
Hydroxychloroquine is used as the active pharmaceutical ingredient in the production of oral hydroxychloroquine medications. This drug is primarily prescribed for the treatment and prevention of malaria, as well as for the management of autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis.
Hydroxychloroquine helps control the symptoms of autoimmune diseases by inhibiting certain inflammatory processes and modulating immune system activity. Additionally, by affecting the metabolism of the malaria parasite, it prevents the replication and progression of the disease. The medication is typically administered orally on a daily basis and is generally well tolerated.
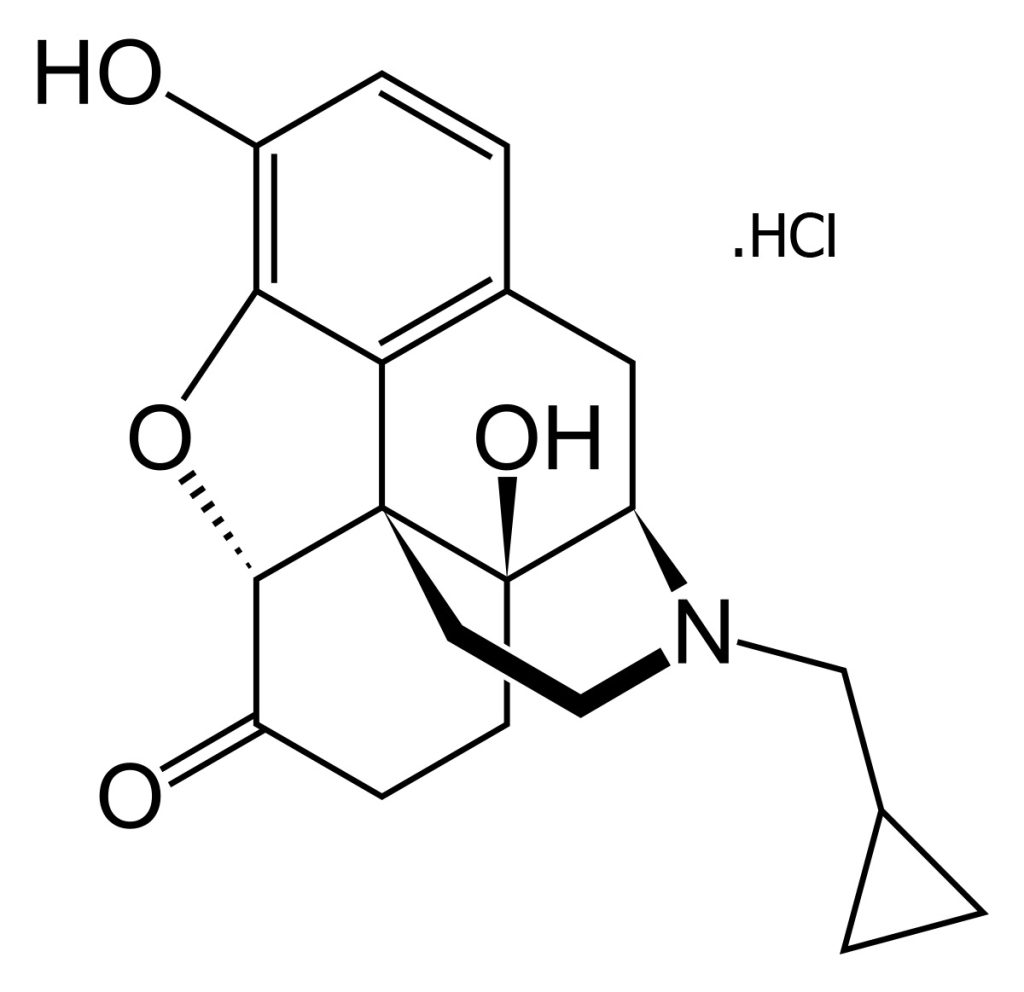
Naltrexone Hydrochloride is used as the active pharmaceutical ingredient in the production of both oral and injectable forms of naltrexone. This medication is primarily prescribed for the treatment of alcohol and opioid dependence.
Naltrexone is an opioid receptor antagonist that helps patients discontinue the use of opioids and alcohol by blocking the effects of these substances and reducing cravings. By binding to opioid receptors in the brain, naltrexone prevents the euphoric effects associated with drug use. The medication is typically administered once daily, and its efficacy in reducing relapse rates has been well establishe
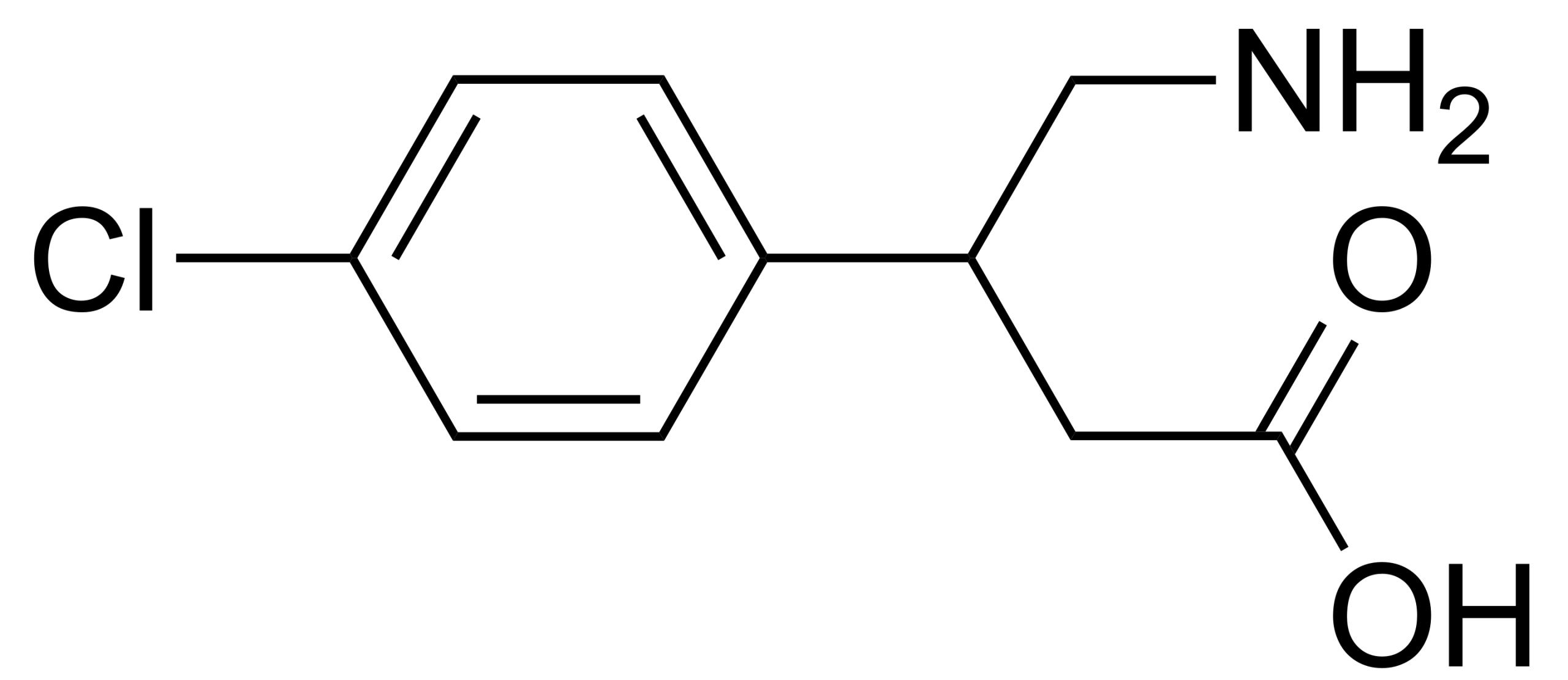
Dihydrocodeine Bitartrate is used as the active pharmaceutical ingredient in oral analgesic and antitussive medications. As an opioid derivative, it is prescribed for the relief of moderate to severe pain, particularly in patients who do not respond adequately to other analgesics. It is also utilized as a cough suppressant in certain syrups and tablets.
Dihydrocodeine acts on opioid receptors in the central nervous system to reduce the sensation of pain and suppress the cough reflex. Compared to morphine, it has a milder effect and generally causes less tolerance and dependence; however, it should still be used with caution. The onset of action typically occurs within one hour of oral administration, with effects lasting approximately 4 to 6 hours.
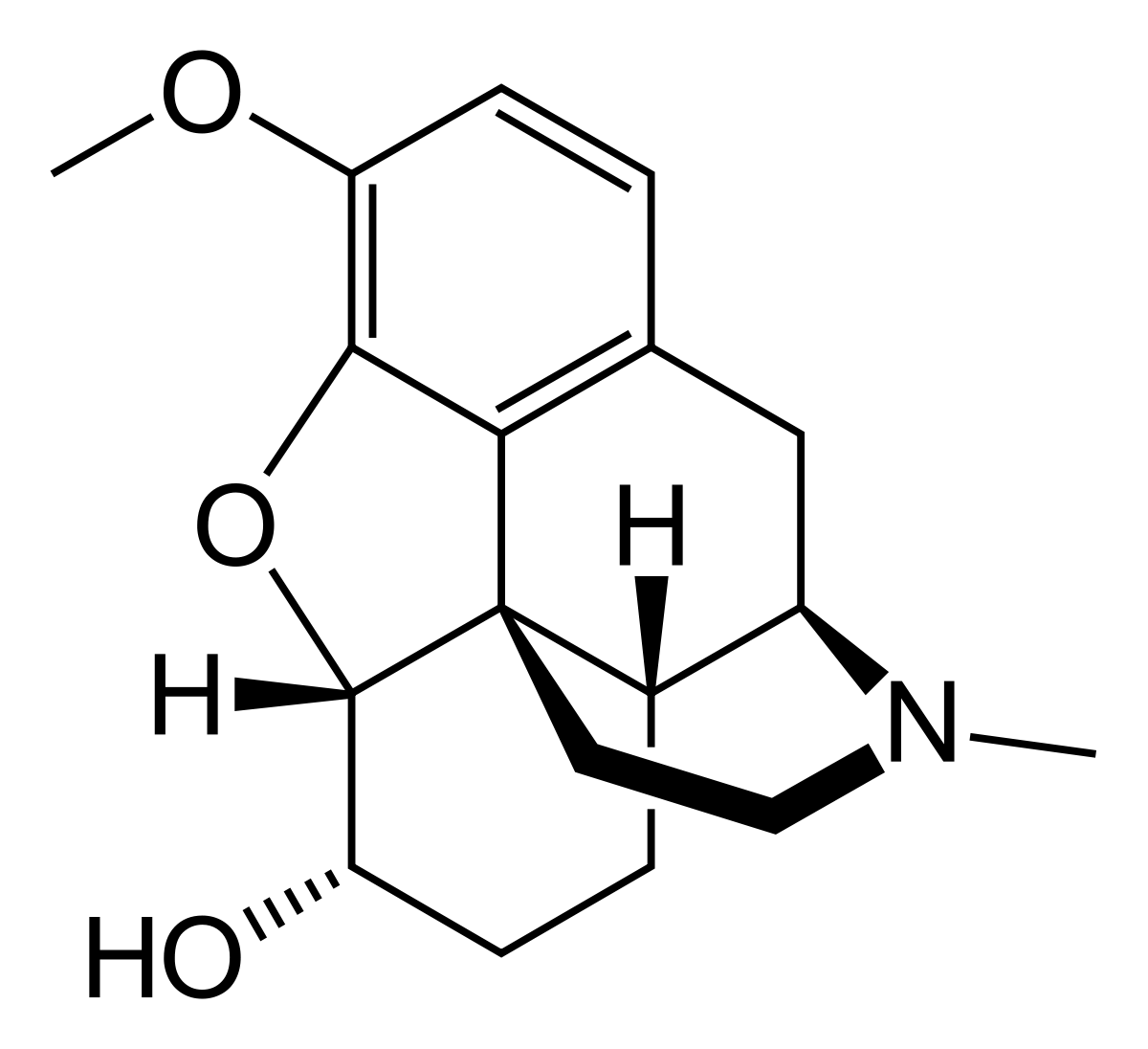
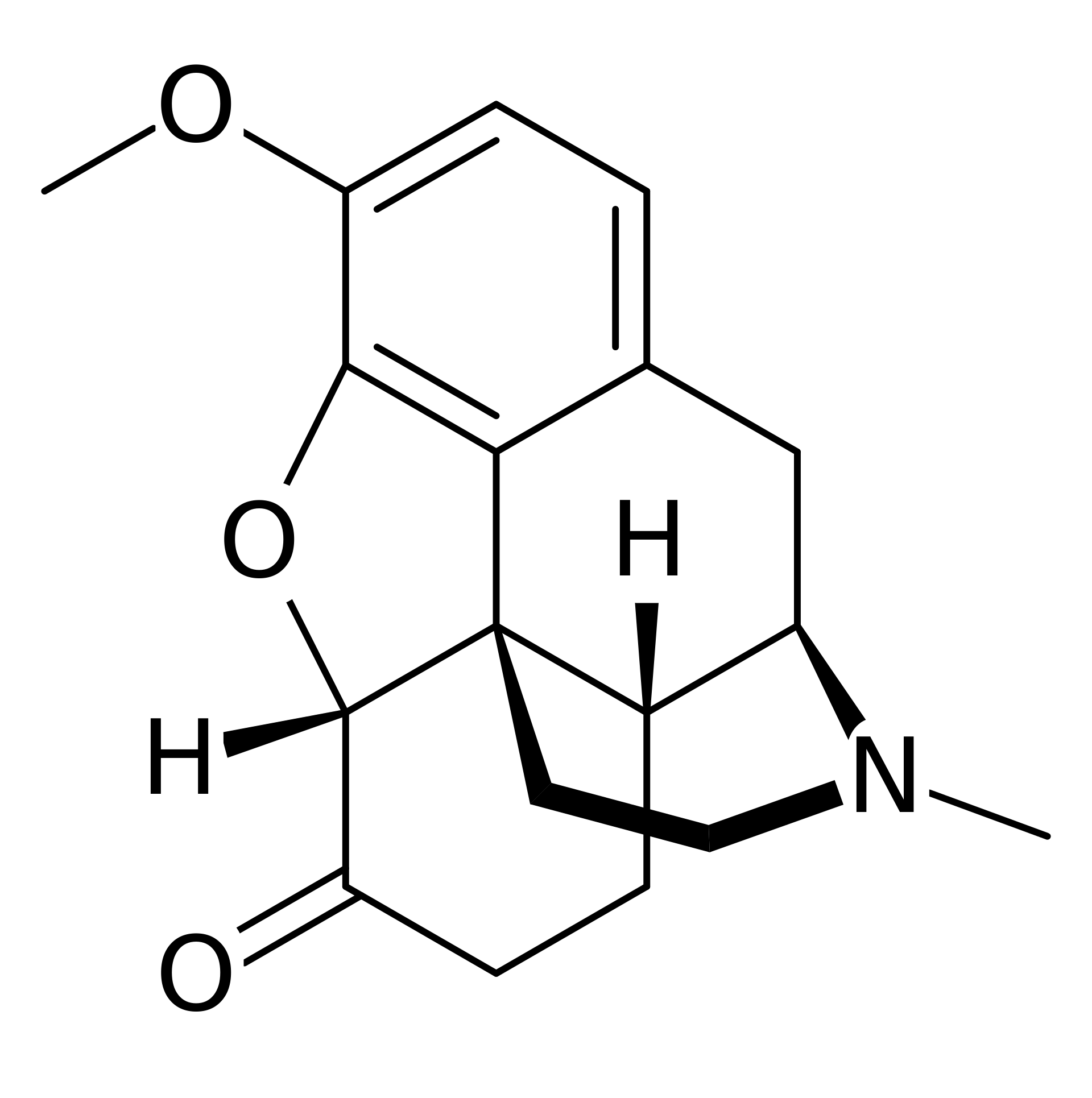
Hydrocodone Bitartrate is used as the primary active ingredient in oral analgesic and antitussive medications. As an opioid derivative, it is commonly prescribed for the relief of moderate to severe pain and for suppressing coughs that are resistant to other treatments. Hydrocodone is often available in combination with acetaminophen or ibuprofen.
Hydrocodone acts on opioid receptors within the central nervous system to reduce pain perception and suppress the cough reflex. Although it is less potent than morphine, it should still be used with caution due to the potential for dependence and tolerance. The onset of action typically occurs within 20 to 30 minutes after oral administration, with effects lasting approximately 4 to 6 hours.
Nalbuphine hydrochloride API, a Level 1 knowledge-based product of Soroush Mana Pharmed, is utilized as the primary active component in the manufacture of injectable nalbuphine hydrochloride. Introduced to the U.S. pharmaceutical market and globally in 1963, nalbuphine is a synthetic phenanthrene opioid agonist-antagonist analgesic. Its analgesic potency is essentially equivalent to morphine on a milligram-for-milligram basis. Nalbuphine injections are used both for anesthesia support prior to surgical procedures and as an analgesic for moderate to severe pain.
While the exact mechanism of action of nalbuphine is not fully understood, it is believed to interact with opioid receptors in the central nervous system. Its opioid antagonist effects are thought to result from competitive inhibition at opioid receptors, although other mechanisms may also be involved.
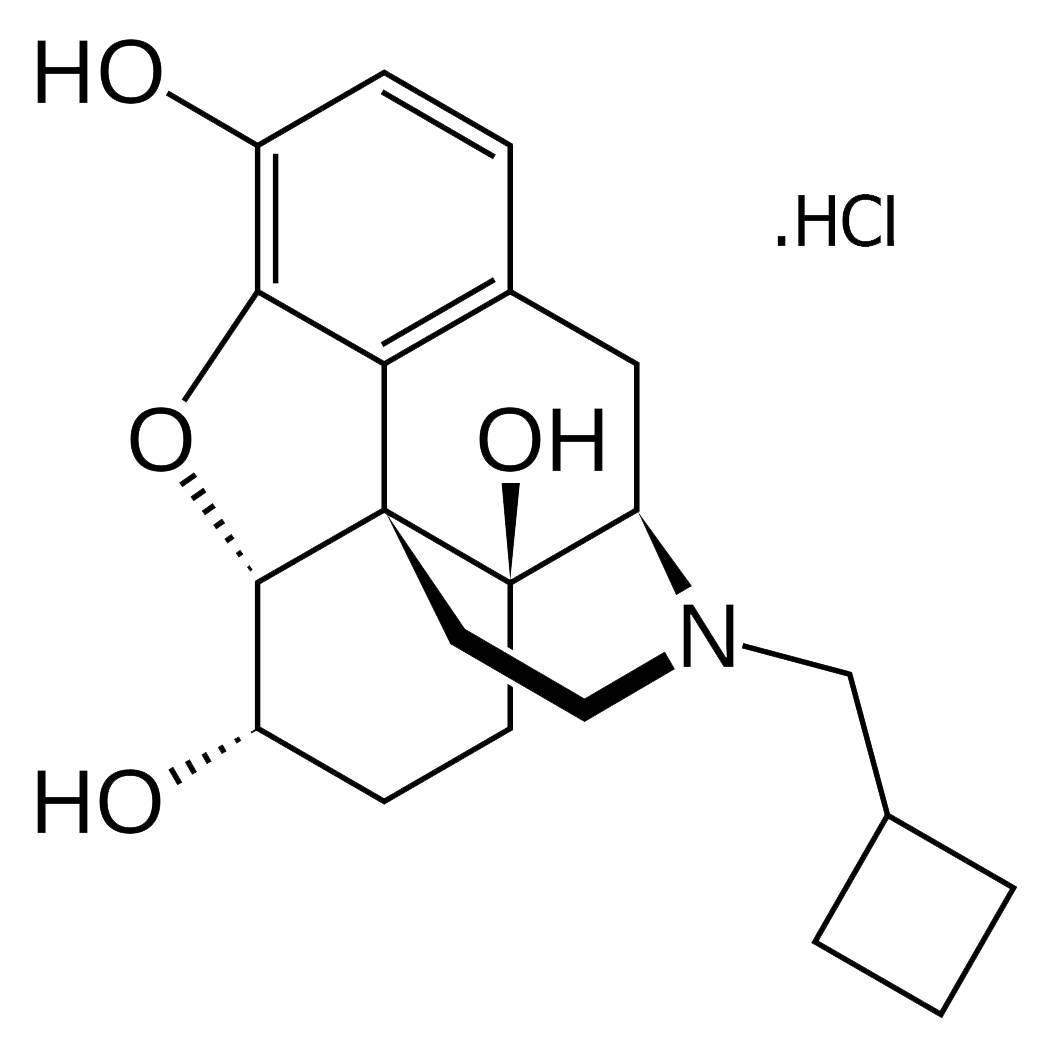
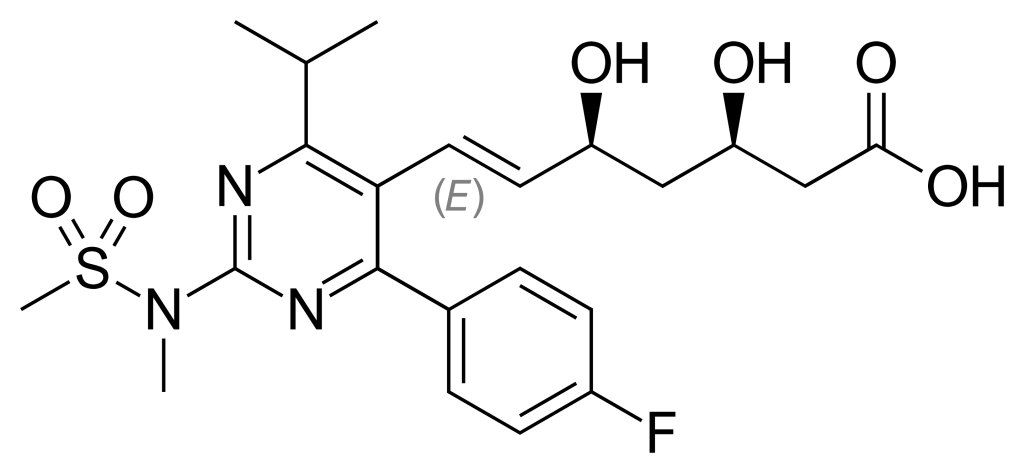
Tramadol Hydrochloride is used as the active pharmaceutical ingredient in both oral and injectable formulations of tramadol. This medication is a centrally acting opioid analgesic, prescribed for the management of moderate to severe pain, including postoperative pain, chronic musculoskeletal pain, and neuropathic pain.
Tramadol alleviates pain by acting on opioid receptors in the brain and inhibiting the reuptake of norepinephrine and serotonin. Compared to other opioids, tramadol has a lower potential for dependence and tolerance; however, there remains a risk of misuse and addiction. The onset of action typically occurs within one hour of oral administration, with effects lasting approximately 4 to 6 hours.

Aripiprazole API is one of the key products of Soroush Mana Pharmed and serves as the main active component in the manufacture of oral aripiprazole medications. Since 2002, aripiprazole has been approved by the U.S. Food and Drug Administration (FDA) for clinical use in the United States.
This medication is primarily indicated for the treatment of schizophrenia and acute manic episodes associated with bipolar disorder. Other uses include adjunctive therapy in major depressive disorder, the management of tic disorders, and the treatment of irritability associated with autism.
Aripiprazole acts as a 5-HT1A receptor antagonist and a partial agonist at the 5-HT2A receptor. While the precise mechanism of action is not fully understood, its therapeutic effects are believed to be mediated through modulation of dopamine and serotonin levels.
Duloxetine hydrochloride API, a Level 1 knowledge-based product of Soroush Mana Pharmed, is utilized as the primary active component in the manufacture of oral duloxetine medication. Approved since 2004 by both the U.S. Food and Drug Administration (FDA) and the European Union, duloxetine belongs to the class of neurological drugs and is prescribed for the treatment of certain mood and neurological disorders, depression, anxiety disorders, neuropathic pain in diabetic patients (diabetic neuropathy), and fibromyalgia.
Duloxetine is a potent serotonin-norepinephrine reuptake inhibitor (SNRI) and a weak dopamine reuptake inhibitor. By regulating the levels of neurotransmitters in the brain (serotonin and norepinephrine), it helps alleviate symptoms of depression. Additionally, it improves symptoms of diabetic neuropathic pain, certain anxiety disorders, and fibromyalgia by modulating serotonin and norepinephrine levels.

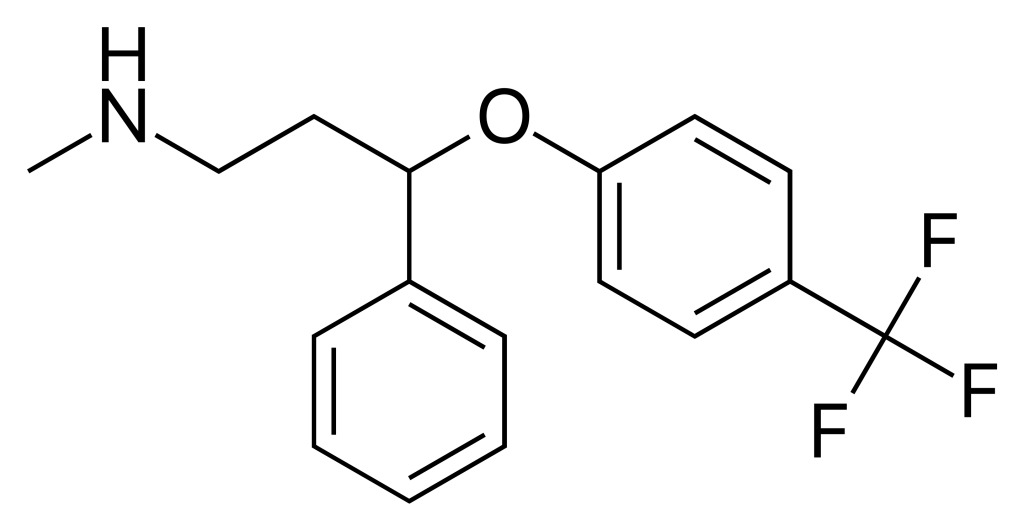
Fluoxetine is used as the active pharmaceutical ingredient in the production of oral fluoxetine medications. This drug is a selective serotonin reuptake inhibitor (SSRI) and is primarily prescribed for the treatment of major depressive disorder, obsessive-compulsive disorder (OCD), generalized anxiety disorder, panic disorder, premenstrual dysphoric disorder (PMDD), and bulimia nervosa.
Fluoxetine works by inhibiting the reuptake of serotonin at nerve terminals in the brain, thereby increasing serotonin levels in the synaptic cleft and improving mood and anxiety symptoms. It is typically administered once daily, and its therapeutic effects may take 2 to 4 weeks to become apparent. Compared to older generations of antidepressants, fluoxetine has fewer side effects and is generally well tolerated by patients.
Fluvoxamine is used as the active pharmaceutical ingredient in the production of oral fluvoxamine medications. This drug belongs to the selective serotonin reuptake inhibitor (SSRI) class and is primarily prescribed for the treatment of obsessive-compulsive disorder (OCD), major depressive disorder, social anxiety disorder, and other anxiety-related conditions.
Fluvoxamine works by inhibiting the reuptake of serotonin at nerve terminals in the brain, thereby increasing serotonin levels in the synaptic cleft. This mechanism helps improve mood, reduce anxiety, and control obsessive thoughts and behaviors. The medication is typically administered once or twice daily, and its therapeutic effects may take 2 to 4 weeks to become apparent.
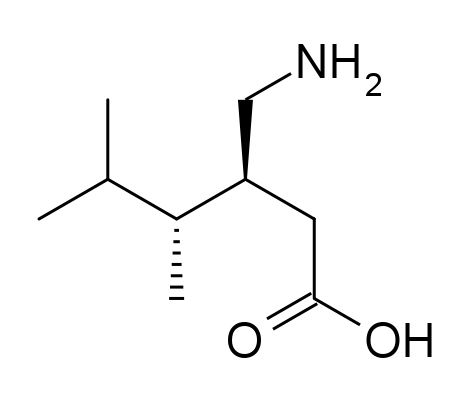
Pregabalin API, recognized as a Level 2 knowledge-based product of Soroush Mana Pharmed, is used as the main active component in the production of oral pregabalin medications. Since 2004, pregabalin has been approved by the U.S. Food and Drug Administration (FDA) for use in the United States.
Pregabalin belongs to the class of antiepileptic drugs, but it is also prescribed for other conditions, such as chronic neuropathic pain associated with diseases like diabetes, shingles (herpes zoster), and fibromyalgia. Additionally, it is used to reduce short-term seizure attacks in a type of epilepsy known as partial (focal) seizures, in combination with other antiepileptic drugs. In this condition, short-term seizures occur due to abnormal electrical activity in cells on one side of the brain, resulting in symptoms localized to specific parts of the body.
Baclofen is utilized as the active pharmaceutical ingredient in both oral and injectable (intrathecal) formulations. It is a centrally acting muscle relaxant primarily prescribed for the treatment of spasticity (muscle stiffness and spasms) resulting from neurological conditions such as multiple sclerosis (MS), spinal cord injuries, cerebral palsy, and other disorders of the central nervous system.
Baclofen acts by stimulating GABA-B receptors in the spinal cord and brain, thereby inhibiting the transmission of excitatory nerve signals and reducing muscle tone and spasticity. It can be administered orally (as tablets) or via intrathecal injection. In addition to reducing muscle spasms, baclofen may also improve motor function and decrease muscle pain in certain patients.
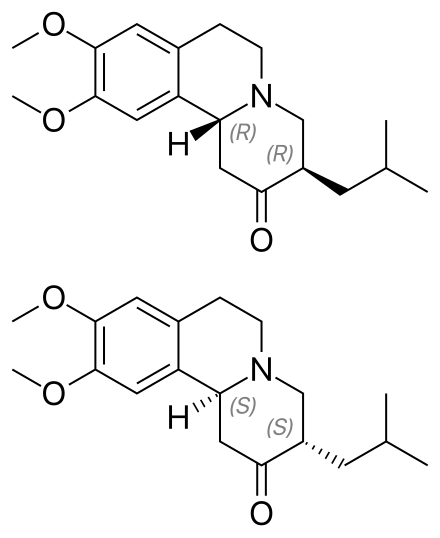
Tetrabenazine API, recognized as a top-tier knowledge-based product of Soroush Mana Pharmed, serves as the primary active component in the manufacture of oral tetrabenazine medication. Approved by the U.S. Food and Drug Administration in 2008, tetrabenazine is a neurological drug indicated for the reduction of involuntary movements in conditions such as Huntington’s disease, hemiballismus, senile chorea, Tourette syndrome, and tardive dyskinesia. Previously, it was also used as an antipsychotic for the treatment of schizophrenia.
Tetrabenazine acts as a central monoamine depletor, exerting its therapeutic effects by reducing monoamine neurotransmitters in the brain, such as dopamine, serotonin, and norepinephrine. e.
Celecoxib is used as the active pharmaceutical ingredient in the production of oral celecoxib medications. This drug is a non-steroidal anti-inflammatory drug (NSAID) from the selective COX-2 inhibitor class, and is primarily prescribed for the treatment of pain and inflammation associated with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and chronic musculoskeletal pain.
Celecoxib works by selectively inhibiting the cyclooxygenase-2 (COX-2) enzyme, thereby reducing the production of inflammatory prostaglandins, which leads to pain relief and decreased inflammation. Unlike non-selective NSAIDs, celecoxib has minimal effect on the COX-1 enzyme, resulting in a lower risk of gastrointestinal side effects such as ulcers and gastrointestinal bleeding. It is usually administered orally, once or twice daily.
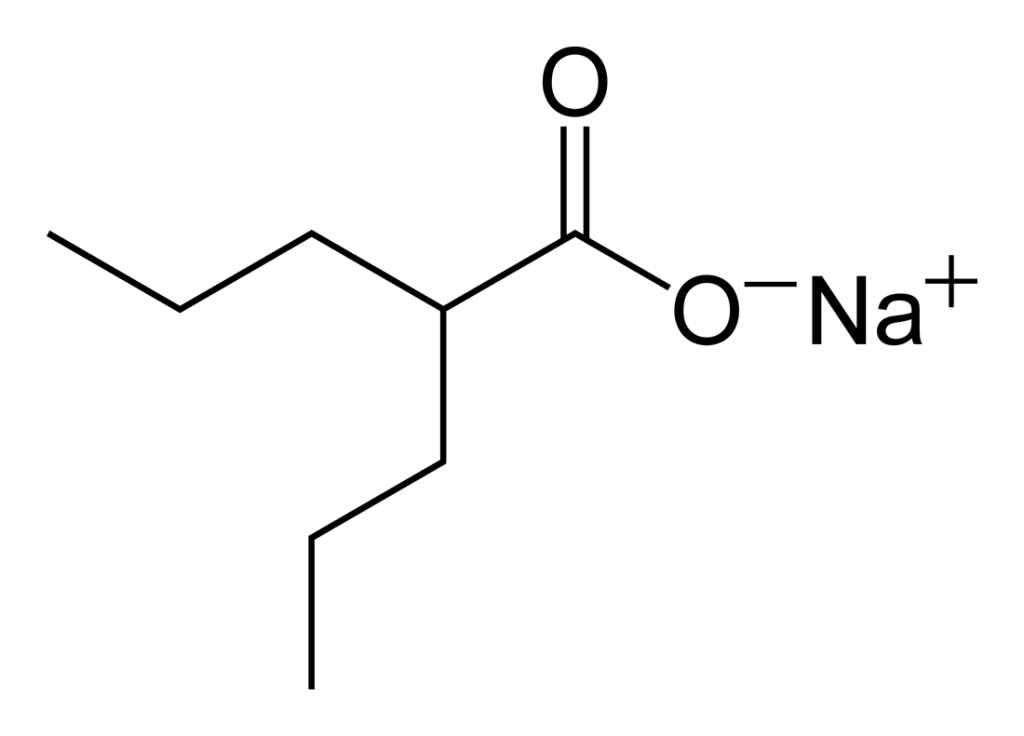
Sodium valproate API, developed as a top-tier knowledge-based product by Soroush Mana Pharmed, serves as the essential and active component in the manufacture of oral sodium valproate pharmaceuticals. Since its approval for clinical use globally in the 1970s, sodium valproate has been classified among antiepileptic drugs and mood stabilizers and is prescribed for the management of various types of epilepsy (including tonic-clonic and partial seizures), bipolar disorder (particularly for manic episodes), and migraine prevention.
Sodium valproate acts by increasing the concentration of gamma-aminobutyric acid (GABA) in the brain, thereby inhibiting overactive neuronal activity and preventing seizures.
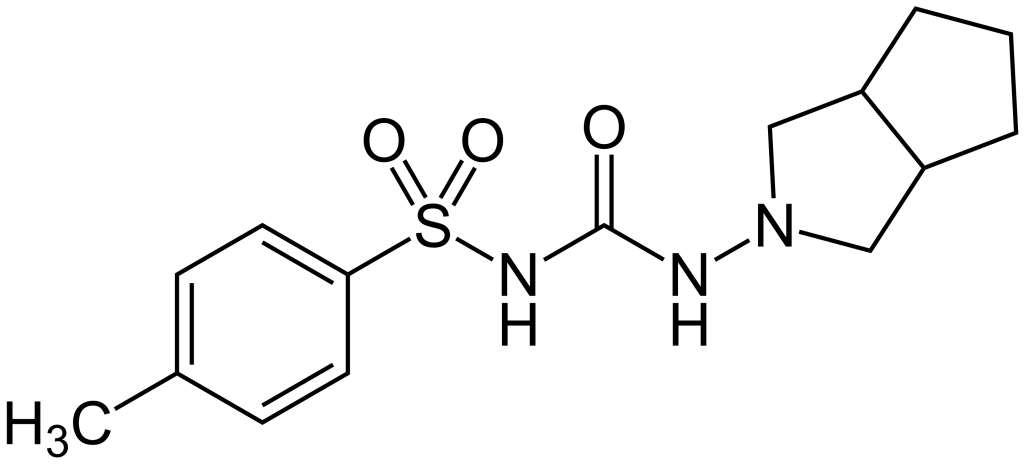
Gliclazide API, produced by Soroush Mana Pharmed, is utilized as the key active component in the manufacture of oral gliclazide medication. Gliclazide belongs to the second generation of sulfonylurea drugs and stimulates pancreatic beta cells to increase insulin secretion. In addition to raising insulin levels, gliclazide also reduces hepatic gluconeogenesis, which can result in an increased number and sensitivity of insulin receptors and lower blood glucose levels. This medication effectively decreases fasting blood sugar, postprandial blood sugar, and glycated hemoglobin (HbA1c) levels.
Repaglinide API, produced by Soroush Mana Pharmed, is utilized as the primary active component in the manufacture of oral repaglinide medication. Repaglinide is an antidiabetic agent from the meglitinide class, prescribed to control blood sugar levels in individuals with type 2 diabetes (non-insulin-dependent diabetes). This non-sulfonylurea hypoglycemic drug acts by inhibiting ATP-dependent potassium channels, depolarizing the cell membrane, and facilitating calcium influx through calcium channels.
By increasing intracellular calcium, repaglinide stimulates pancreatic beta cells to release more insulin.
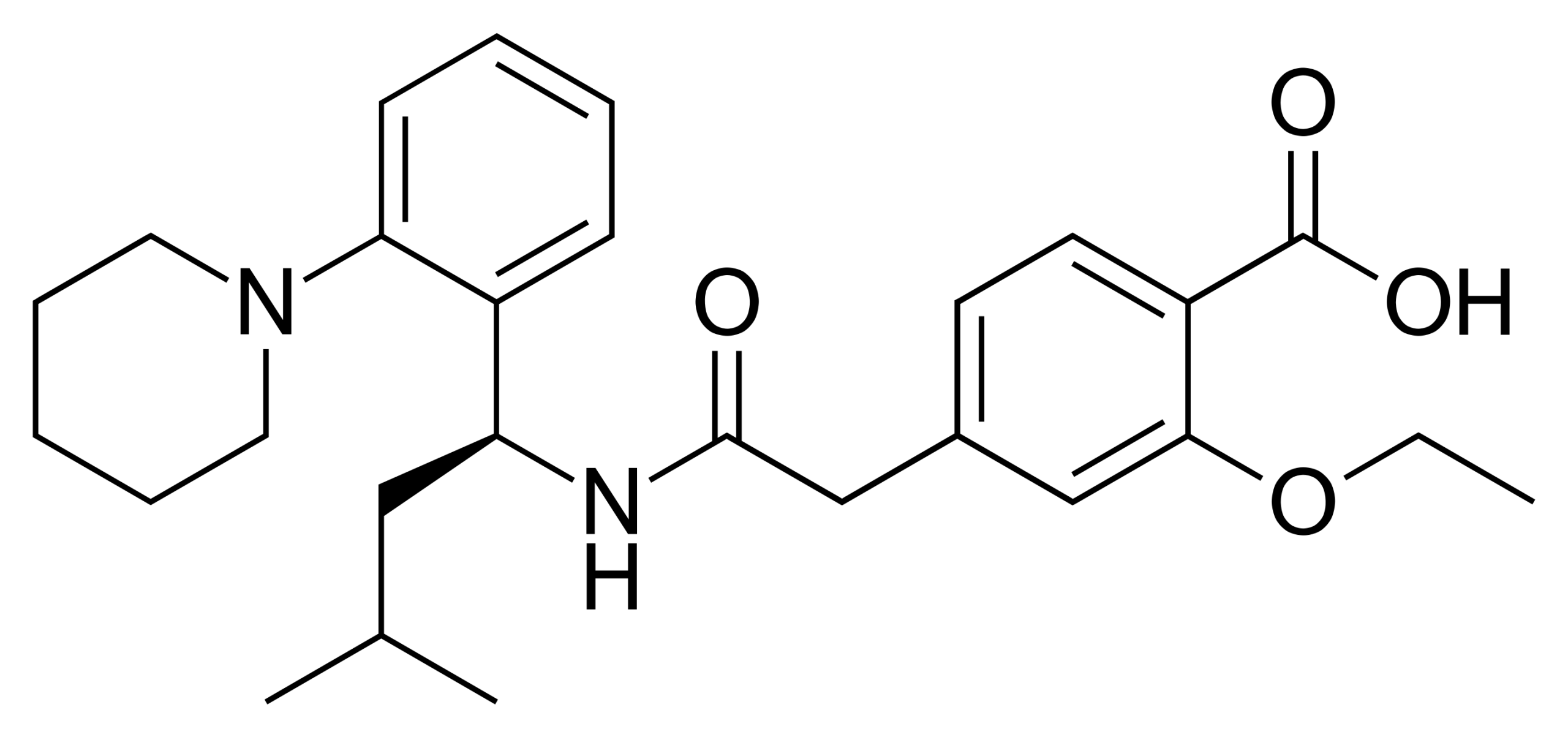

Rosuvastatin Calcium is used as the active pharmaceutical ingredient (API) in the production of oral rosuvastatin medications. This drug is indicated for the treatment of various lipid disorders, including primary hypercholesterolemia, mixed dyslipidemia, and familial hyperlipidemia. It is also recommended for reducing the risk of cardiovascular events in high-risk patients.
Rosuvastatin is one of the most potent statins and works by inhibiting the HMG-CoA reductase enzyme, effectively lowering LDL cholesterol and triglycerides while increasing HDL cholesterol. In addition to its lipid-lowering effects, rosuvastatin reduces vascular inflammation and stabilizes atherosclerotic plaques.
Telmisartan is used as the active pharmaceutical ingredient (API) in the production of oral telmisartan medications for the management of hypertension, renal protection in diabetic patients with nephropathy, and reduction of cardiovascular event risk. It is also indicated for use in patients with heart failure.
Telmisartan belongs to the class of angiotensin II receptor blockers (ARBs) and works by inhibiting the effects of angiotensin II, leading to vasodilation and reduced blood pressure. In addition to controlling hypertension, telmisartan plays a crucial role in preventing kidney damage caused by diabetes and in reducing the risk of stroke and heart attack in high-risk patients.
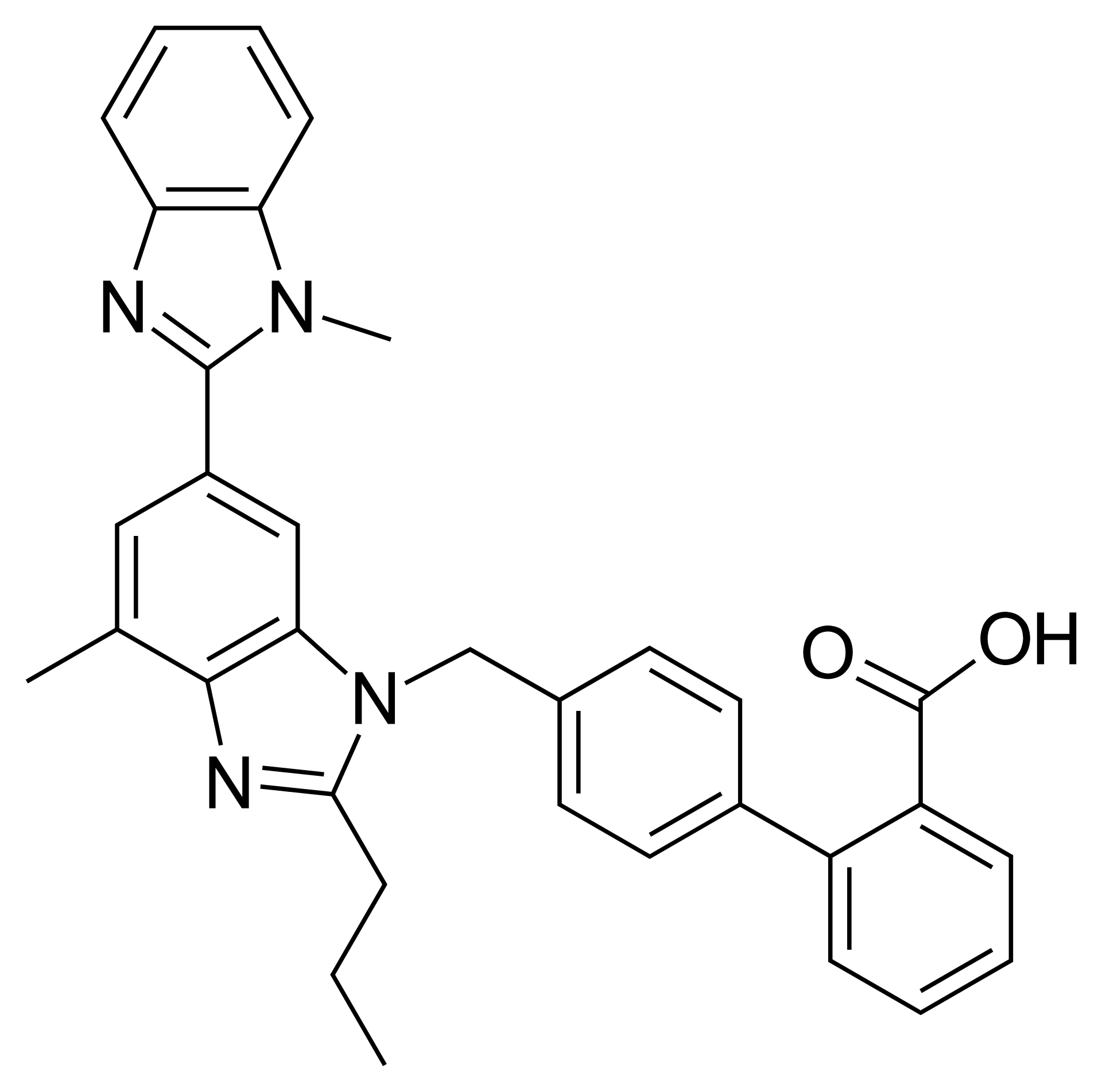

The sacubitril-valsartan active pharmaceutical ingredient is utilized as the main active component in the manufacture of oral sacubitril-valsartan medication. Approved in 2015 by both the United States and the European Union, this combination drug is indicated for patients with chronic heart failure. Sacubitril-valsartan reduces the need for hospitalization due to severe heart failure and lowers mortality rates associated with the condition.
This product combines sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor type 1 blocker. The combination is sometimes referred to as an “angiotensin receptor-neprilysin inhibitor (ARNI).”

Famotidine is used as the active pharmaceutical ingredient in both oral and injectable famotidine medications. This drug is an H2 histamine receptor antagonist, primarily employed to reduce gastric acid secretion. Famotidine is mainly prescribed for the treatment of gastric and duodenal ulcers, gastroesophageal reflux disease (GERD), acid-related indigestion, and for the prevention of gastrointestinal bleeding in hospitalized patients.
Famotidine works by inhibiting H2 receptors on the parietal cells of the stomach, thereby decreasing gastric acid secretion. Compared to previous generations of H2 antagonists, famotidine offers higher efficacy and fewer side effects..
Pantoprazole is used as the active pharmaceutical ingredient in both oral and injectable formulations of pantoprazole. As a member of the proton pump inhibitor (PPI) class, this medication is primarily prescribed for the treatment of gastroesophageal reflux disease (GERD), gastric and duodenal ulcers, Zollinger-Ellison syndrome, and other conditions associated with excessive gastric acid secretion.
Pantoprazole works by irreversibly inhibiting the proton pump (H+/K+ ATPase) in the gastric parietal cells, resulting in a significant reduction in gastric acid secretion. This action helps alleviate symptoms of heartburn, promotes the healing of gastrointestinal ulcers, and prevents their recurrence. The onset of action typically occurs within a few hours, with the full therapeutic effect achieved over several days.
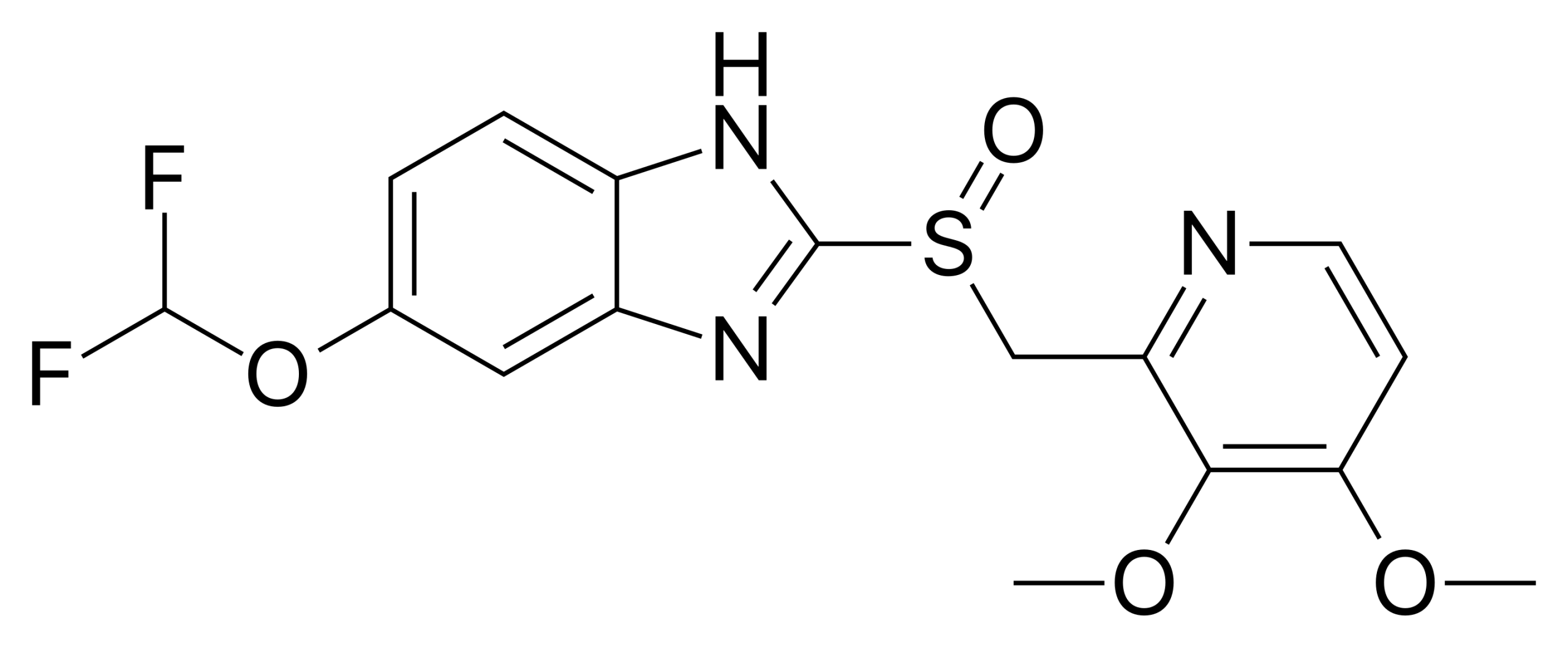
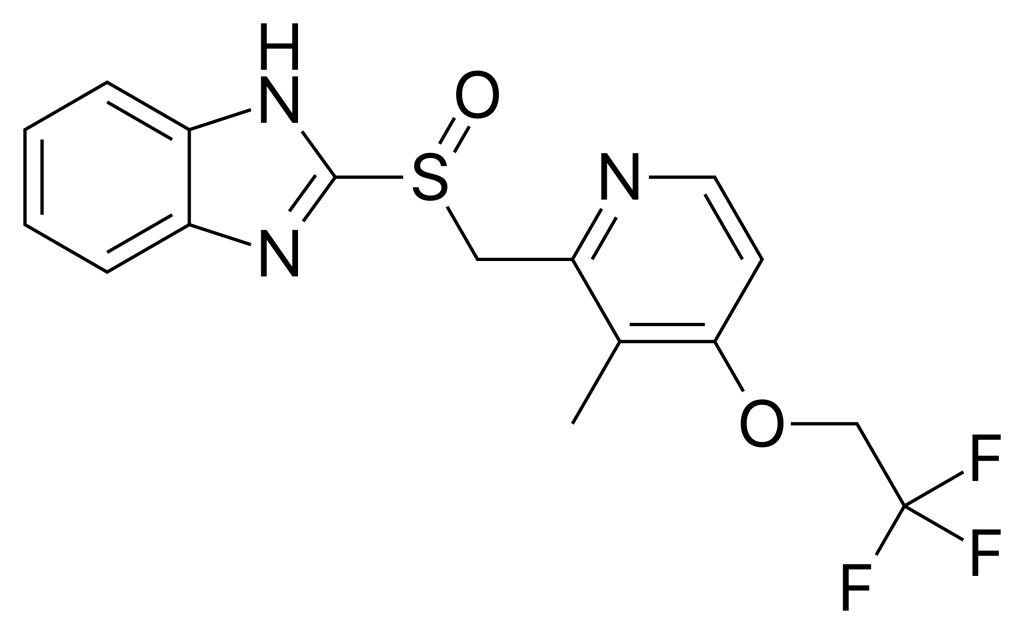
Lansoprazole, marketed under the brand name Prevacid, is a leading drug in the proton pump inhibitor (PPI) class, playing a crucial role in reducing gastric acid secretion. Approved by the U.S. Food and Drug Administration (FDA) since 1995, lansoprazole is widely prescribed around the world for the management of acid-related gastrointestinal diseases.
The primary indications for lansoprazole include the treatment of gastric and duodenal ulcers, GERD, Zollinger-Ellison syndrome, and the prevention of NSAID-induced gastric ulcers. Additionally, lansoprazole is used in combination with antibiotics for the eradication of Helicobacter pylori infections. Lansoprazole acts by irreversibly inhibiting the H+/K+ ATPase enzyme, commonly known as the proton pump, located in the parietal cells of the stomach lining.
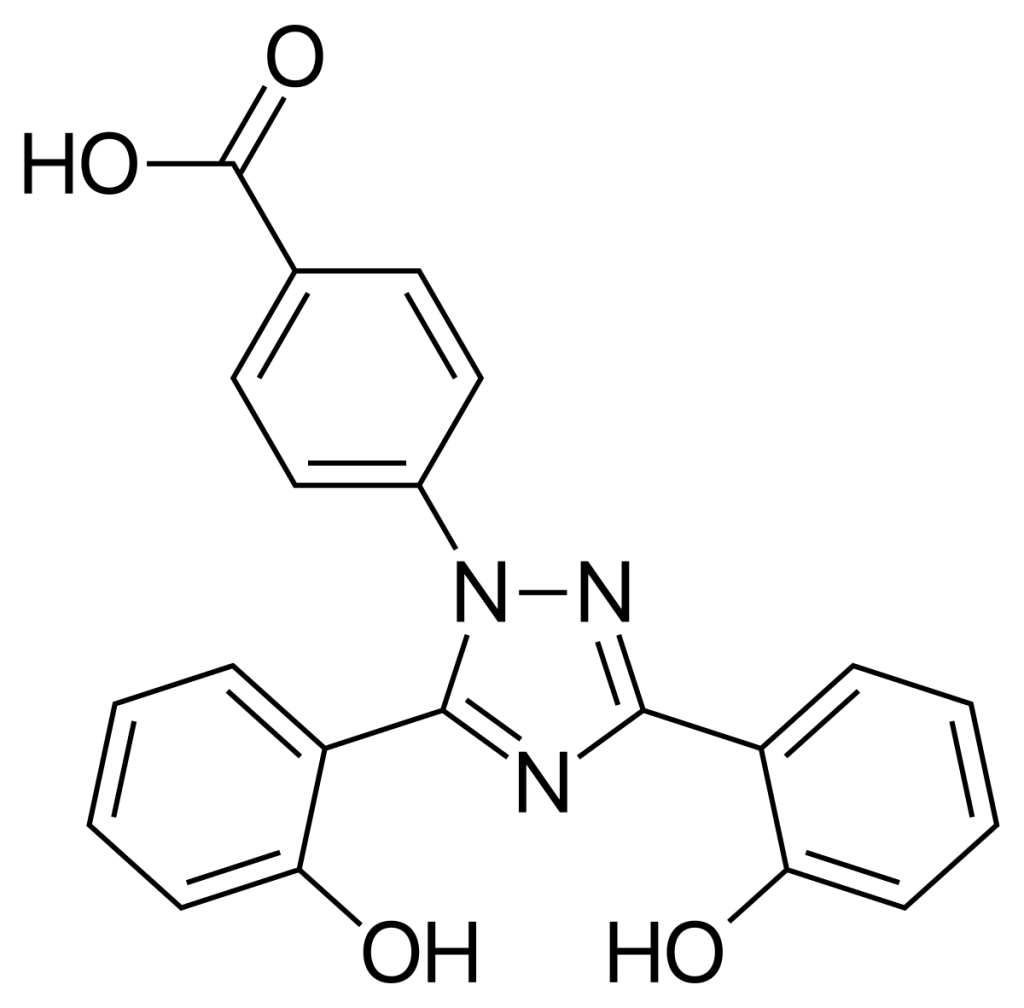
Deferasirox API, developed as a Level 2 knowledge-based product by Soroush Mana Pharmed, is utilized as the key active component in the manufacture of oral deferasirox medication. Approved by the U.S. Food and Drug Administration (FDA) in 2005, deferasirox is an iron chelating agent that promotes the excretion of excess iron from the body. It is primarily prescribed in cases of chronic iron overload resulting from long-term blood transfusions or conditions such as thalassemia, where iron toxicity becomes a concern.
Two molecules of deferasirox have the capacity to bind to a single iron atom, thereby reducing serum iron concentration. Iron excretion through this medication occurs mainly via the feces, with minimal renal elimination through the urine.