
Aripiprazole API is one of the key products of Soroush Mana Pharmed and serves as the main active component in the manufacture of oral aripiprazole medications. Since 2002, aripiprazole has been approved by the U.S. Food and Drug Administration (FDA) for clinical use in the United States.
This medication is primarily indicated for the treatment of schizophrenia and acute manic episodes associated with bipolar disorder. Other uses include adjunctive therapy in major depressive disorder, the management of tic disorders, and the treatment of irritability associated with autism.
Aripiprazole acts as a 5-HT1A receptor antagonist and a partial agonist at the 5-HT2A receptor. While the precise mechanism of action is not fully understood, its therapeutic effects are believed to be mediated through modulation of dopamine and serotonin levels.
Duloxetine hydrochloride API, a Level 1 knowledge-based product of Soroush Mana Pharmed, is utilized as the primary active component in the manufacture of oral duloxetine medication. Approved since 2004 by both the U.S. Food and Drug Administration (FDA) and the European Union, duloxetine belongs to the class of neurological drugs and is prescribed for the treatment of certain mood and neurological disorders, depression, anxiety disorders, neuropathic pain in diabetic patients (diabetic neuropathy), and fibromyalgia.
Duloxetine is a potent serotonin-norepinephrine reuptake inhibitor (SNRI) and a weak dopamine reuptake inhibitor. By regulating the levels of neurotransmitters in the brain (serotonin and norepinephrine), it helps alleviate symptoms of depression..

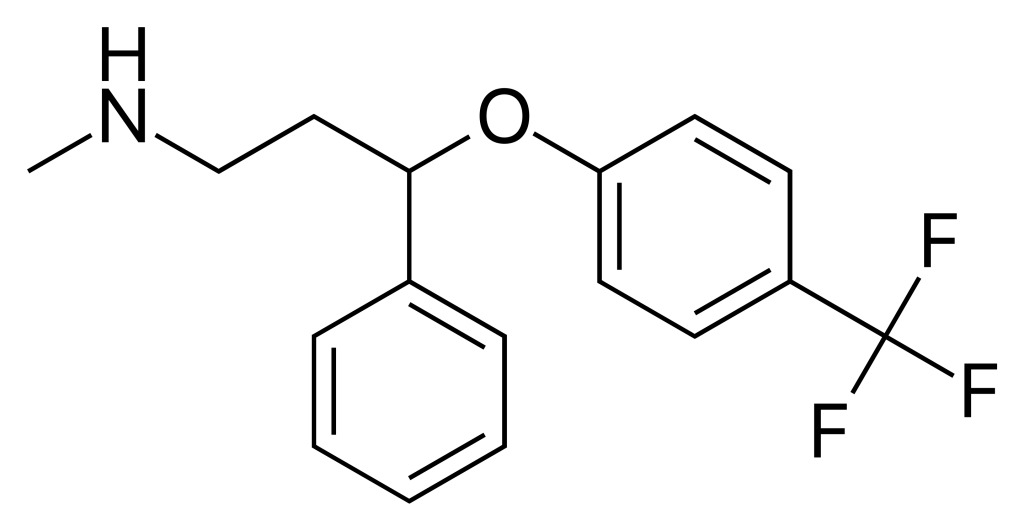
Fluoxetine is used as the active pharmaceutical ingredient in the production of oral fluoxetine medications. This drug is a selective serotonin reuptake inhibitor (SSRI) and is primarily prescribed for the treatment of major depressive disorder, obsessive-compulsive disorder (OCD), generalized anxiety disorder, panic disorder, premenstrual dysphoric disorder (PMDD), and bulimia nervosa.
Fluoxetine works by inhibiting the reuptake of serotonin at nerve terminals in the brain, thereby increasing serotonin levels in the synaptic cleft and improving mood and anxiety symptoms. It is typically administered once daily, and its therapeutic effects may take 2 to 4 weeks to become apparent. Compared to older generations of antidepressants, fluoxetine has fewer side effects and is generally well tolerated by patients.
Fluvoxamine is used as the active pharmaceutical ingredient in the production of oral fluvoxamine medications. This drug belongs to the selective serotonin reuptake inhibitor (SSRI) class and is primarily prescribed for the treatment of obsessive-compulsive disorder (OCD), major depressive disorder, social anxiety disorder, and other anxiety-related conditions.
Fluvoxamine works by inhibiting the reuptake of serotonin at nerve terminals in the brain, thereby increasing serotonin levels in the synaptic cleft. This mechanism helps improve mood, reduce anxiety, and control obsessive thoughts and behaviors. The medication is typically administered once or twice daily, and its therapeutic effects may take 2 to 4 weeks to become apparent.
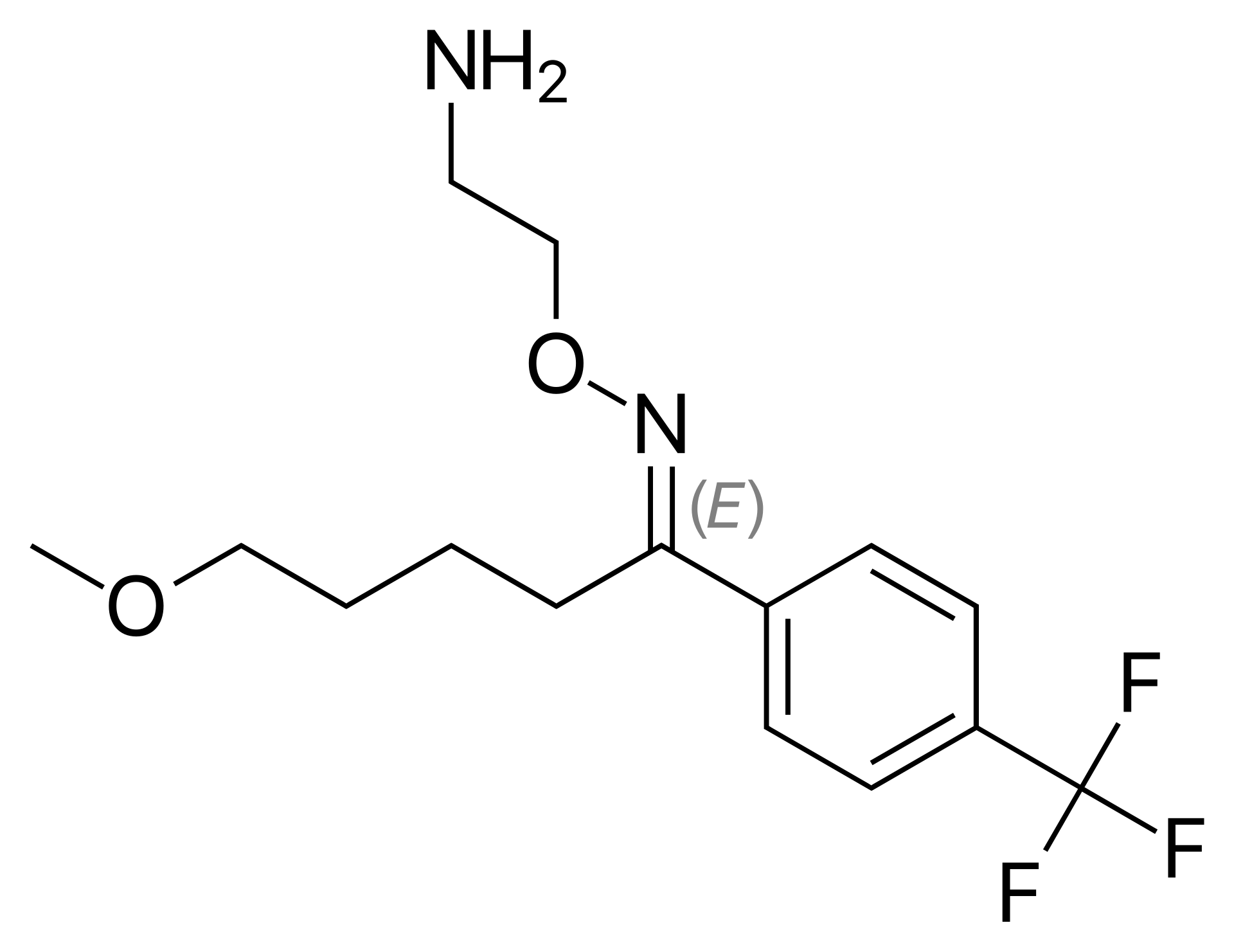
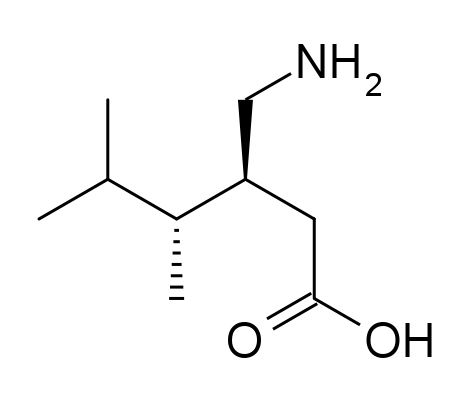
Pregabalin API, recognized as a Level 2 knowledge-based product of Soroush Mana Pharmed, is used as the main active component in the production of oral pregabalin medications. Since 2004, pregabalin has been approved by the U.S. Food and Drug Administration (FDA) for use in the United States.
Pregabalin belongs to the class of antiepileptic drugs, but it is also prescribed for other conditions, such as chronic neuropathic pain associated with diseases like diabetes, shingles (herpes zoster), and fibromyalgia. Additionally, it is used to reduce short-term seizure attacks in a type of epilepsy known as partial (focal) seizures, in combination with other antiepileptic drugs. In this condition, short-term seizures occur due to abnormal electrical activity in cells on one side of the brain, resulting in symptoms localized to specific parts of the body. .
Baclofen is utilized as the active pharmaceutical ingredient in both oral and injectable (intrathecal) formulations. It is a centrally acting muscle relaxant primarily prescribed for the treatment of spasticity (muscle stiffness and spasms) resulting from neurological conditions such as multiple sclerosis (MS), spinal cord injuries, cerebral palsy, and other disorders of the central nervous system.
Baclofen acts by stimulating GABA-B receptors in the spinal cord and brain, thereby inhibiting the transmission of excitatory nerve signals and reducing muscle tone and spasticity. It can be administered orally (as tablets) or via intrathecal injection. In addition to reducing muscle spasms, baclofen may also improve motor function and decrease muscle pain in certain patients.
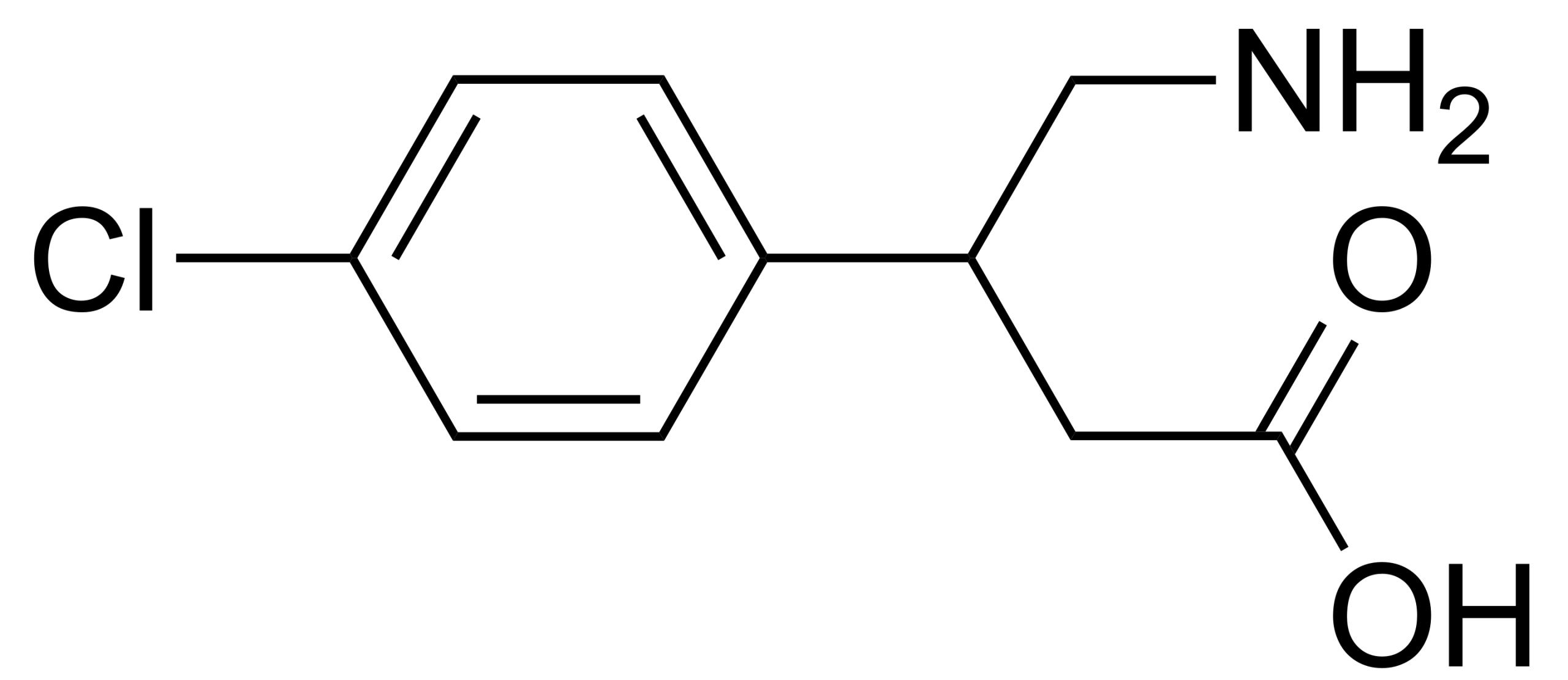
Tetrabenazine API, recognized as a top-tier knowledge-based product of Soroush Mana Pharmed, serves as the primary active component in the manufacture of oral tetrabenazine medication. Approved by the U.S. Food and Drug Administration in 2008, tetrabenazine is a neurological drug indicated for the reduction of involuntary movements in conditions such as Huntington’s disease, hemiballismus, senile chorea, Tourette syndrome, and tardive dyskinesia. Previously, it was also used as an antipsychotic for the treatment of schizophrenia.
Tetrabenazine acts as a central monoamine depletor, exerting its therapeutic effects by reducing monoamine neurotransmitters in the brain, such as dopamine, serotonin, and norepinephrine. It works through the reversible inhibition of VMAT2 receptors, thereby decreasing the uptake of monoamines by synaptic vesicles and subsequently lowering monoamine storage.
Celecoxib is used as the active pharmaceutical ingredient in the production of oral celecoxib medications. This drug is a non-steroidal anti-inflammatory drug (NSAID) from the selective COX-2 inhibitor class, and is primarily prescribed for the treatment of pain and inflammation associated with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and chronic musculoskeletal pain.
Celecoxib works by selectively inhibiting the cyclooxygenase-2 (COX-2) enzyme, thereby reducing the production of inflammatory prostaglandins, which leads to pain relief and decreased inflammation. Unlike non-selective NSAIDs, celecoxib has minimal effect on the COX-1 enzyme, resulting in a lower risk of gastrointestinal side effects such as ulcers and gastrointestinal bleeding. It is usually administered orally, once or twice daily.
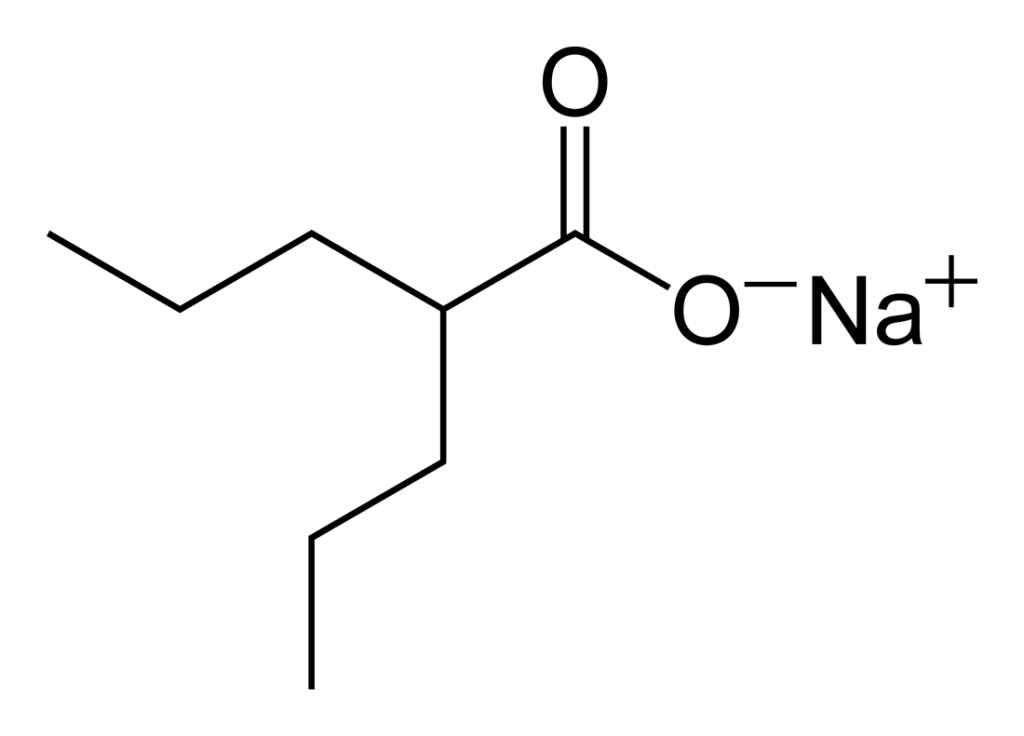
Sodium valproate API, developed as a top-tier knowledge-based product by [Company Name], serves as the essential and active component in the manufacture of oral sodium valproate pharmaceuticals. Since its approval for clinical use globally in the 1970s, sodium valproate has been classified among antiepileptic drugs and mood stabilizers and is prescribed for the management of various types of epilepsy (including tonic-clonic and partial seizures), bipolar disorder (particularly for manic episodes), and migraine prevention.